Access
The NICB is available to bona fide researchers for COVID-19 research in the public interest, in accordance with the NICB Sample and Data Access Policy. All researchers who wish to access the NICB must first register with NICB by completing the registration form
The NICB wishes to ensure fair and efficient access to NICB samples and data to ensure the widest possible usage, whilst ensuring that such access and usage is consistent with the informed consent provided by the participants, and that the research proposal is in the wider public interest (including being scientifically valuable, lawful, and ethically sound).
Research projects will only be approved for access if they align with one of NICB’s Strategic Research Prioritisation Areas. These are currently Long Covid, Therapeutics and Vaccine Response.
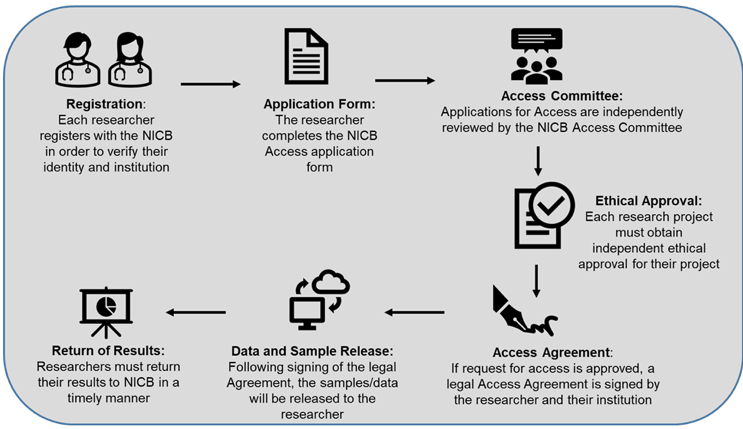
Steps to Access
Collaborate
Are you interested in collaborating with NICB? If so, please complete this form and we will get back to you.
Current Collaborations
Samples
Summary information on samples and data currently stored in the NICB
Participant cohort types:
Acute COVID-19
Long COVID
Controls
Biological samples:
Serum
Plasma
Peripheral Blood Mononuclear Cells
Citrated Plasma
Urine
Data:
The NICB collects the following types of data:
Healthcare information: from your hospital chart, hospital electronic patient record, other hospital databases. Examples: treatments, test results, images/scans
Socio demographic information: Examples: ethnicity, education, living conditions.
Questionnaires: from standard questionnaires that can be compared with other studies internationally.
The NICB Codebook is available here.